- A step-by-step explanation of how to draw the Lewis dot structure for Mg (Magnesium). I show you where Magnesium is on the periodic table and how to determi.
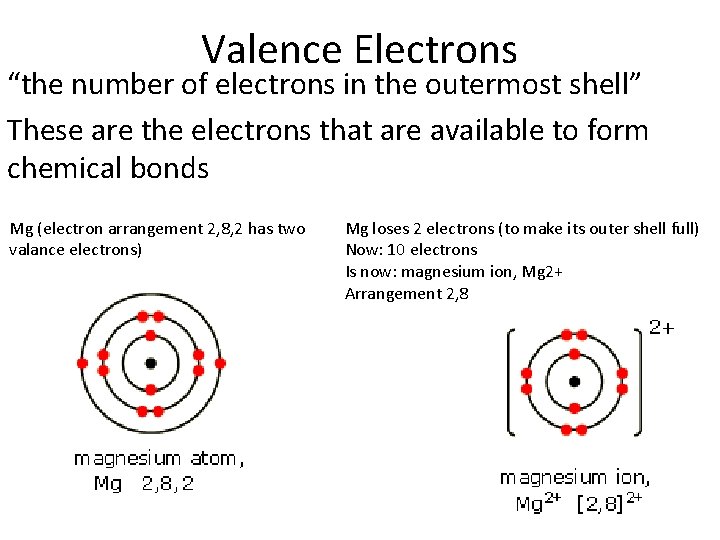
- The electron configuration of Magnesium is 1s22s2p63s2 The 1s22s22p6 is the electron configuration of Neon N e10 This first 10 electrons are in a stable configuration leaving only 2 valance electrons. The 3s2 electrons. So the charge on Magnesium as an ion is usually +2 wen it loses the two 3s2 electrons.
- Magnesium Valence Electrons Google
- Beryllium Magnesium Valence Electrons
- Element Magnesium Valence Electrons
- Magnesium Valence Electrons Outermost Shell
Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Bonetown activation code crack. This is an ionic bond. Magnesium, found in group 2, has 2 valence electrons and needs to release them to become stable. Sulfur, found in group 16, has 6 valence electrons and takes two from.
.jpg)
The key difference between aluminum and magnesium is that the aluminum is a corrosion resistant metal whereas magnesium is not.
Magnesium and aluminum are two chemical elements that we can categorize as metals in the periodic table. Both are naturally occurring metals in different mineral forms. There are many applications of these chemical elements as metals due to their favourable properties.
CONTENTS
1. Overview and Key Difference
2. What is Aluminum
3. What is Magnesium
4. Side by Side Comparison – Aluminum vs Magnesium in Tabular Form
5. Summary
What is Aluminum?
Magnesium Valence Electrons Google
Aluminum or Al is an element in group 3 and period 3 and has the atomic number of 13. The electron configuration of Al is 1s2 2s2 2p6 3s2 3p1. Moreover, it is a silvery white solid, and it is the most abundant metal in the earth crust. It is not soluble in water at room temperature.
Furthermore, the atomic weight of aluminum is 27 g mol-1, and it is a light weighted, durable metal. It doesn’t easily ignite. Also, since this metal is too reactive to stay in its free form, naturally it occurs in mineral forms. Besides, the main aluminum containing mineral is bauxite. Large bauxite ores are located in Australia, Brazil, Jamaica and Guinea. Also, aluminium is in the mineral forms such as cryolite, beryl, garnet, etc.
Due to the low density and resistance to corrosion, manufacturers use aluminum largely in automobiles and other vehicles manufacturing, construction, paints, for household items, packaging etc.
What is Magnesium?
Beryllium Magnesium Valence Electrons
Magnesium is the 12th element in the periodic table. It is in the alkaline earth metal group and the 3rd period. We can denote this metal as Mg. Magnesium is one of the most abundant molecules in the earth. It is an essential element in macro level for plants and animals.
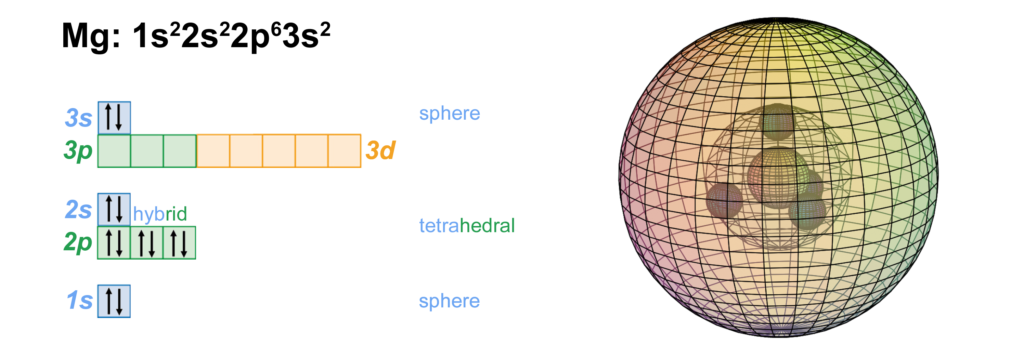
Magnesium has the electron configuration of 1s2 2s2 2p6 3s2. Since there are two electrons in the outer most orbital, magnesium likes to donate that electron to another more electronegative atom and form a +2 charge ion. The atomic weight of Mg is about 24 g mol-1, and it is a lightweight metal, but strong metal.
Nature
Moreover, it is a crystalline solid with a silvery colour. But, it is highly reactive with oxygen; thus, forms a magnesium oxide (MgO) layer when exposed to normal air, which is dark in colour. And, this MgO layer acts as a protective layer. Therefore, naturally, we cannot find this metal as a pure element. When we burn the free magnesium metal, it gives a characteristic sparkling white flame.
Figure 02: Thin Magnesium Films
Also, this metal is highly soluble in water, and reacts with water at room temperature, releasing hydrogen gas bubbles. Furthermore, it also reacts well with most acids and produces MgCl2 and H2 gas. Magnesium largely occurs in seawater and minerals like dolomite, magnesite, carnallite, talc, etc. We can extract this metal from seawater by adding calcium hydroxide. It forms magnesium hydroxide. There, we need to filter the precipitated magnesium hydroxide, and then we need to make it react with HCl to produce MgCl2 again. After that, using electrolysis of magnesium chloride, we can separate the metal at the cathode.
Hbo now for mac. More importantly, magnesium is useful in organic reactions (Grignard reagent), and many other laboratory reactions. Moreover, Mg compounds are incorporated into food, fertilizers and culture media, since it is an essential element for the growth and development of organisms.
What is the Difference Between Aluminum and Magnesium?
Aluminum is a metal having the atomic number 13 and chemical symbol Al and Magnesium is a metal having the atomic number 12 and chemical symbol Mg. The key difference between aluminum and magnesium is that the aluminum is a corrosion resistant metal whereas the magnesium is not. Moreover, there are three valence electrons of aluminum. Thus, it forms +3 cation while magnesium has two valence electrons and can make +2 metal cation. Therefore, this makes another difference between aluminum and magnesium.
Element Magnesium Valence Electrons
An additional difference between aluminum and magnesium is that the aluminum is not soluble in water at room temperature whereas magnesium is highly soluble in water, and reacts with water at room temperature. Apart from that, aluminum does not easily ignite, but magnesium does.
More differences are tabulated in the infographic on the difference between aluminum and magnesium. Best app for mac cleaning 2018.
Summary – Aluminum vs Magnesium
Magnesium and aluminum are metals that have a somewhat similar appearance. However, they are two different metals. The key difference between aluminum and magnesium is that the aluminum is a corrosion resistant metal whereas magnesium is not.
Reference:
1. Helmenstine, Anne Marie, Ph.D. “Aluminum or Aluminium?” ThoughtCo, Sep. 10, 2018. Available here
2. “Magnesium.” National Center for Biotechnology Information. PubChem Compound Database, U.S. National Library of Medicine. Available here
Image Courtesy:


1.”2775895″by HeungSoon (CC0) via pixabay
2.”Magnesium”By Maral10 – Own work, (Public Domain) via Commons Wikimedia
Magnesium Valence Electrons Outermost Shell
Related posts:
